Lecture
In the development of thermodynamics in the XIX century, you can identify three periods. The first is associated with the name of Carnot, who in 1824. in the book "Reflections on the driving force of fire" essentially formulated the first and second principles of thermodynamics. In his discourse, Carnot relied on the hypothesis of the existence of caloric acid * , but managed to come to the right conclusions. The second period continued approximately until the middle of the 19th century and was associated with the development of thermodynamics by outstanding European physicists. Let us briefly discuss their biographies and the contribution they made to the development of the new science. Englishman J. Joel (1818-1889) was born in Manchester to the family of a brewer. Experienced in the 40s, Joule discovered that heat is not conserved. He introduced the concept of mechanical heat equivalent; made sure that the heat is not a substance like water. However, the nature of the warmth for him was not clear. Another Englishman, W. Thomson, or Lord Kelvin (1824-1907), was born in Belfast, at the age of 22 he received the chair of natural philosophy in Glasgow. In 1847, in Oxford, Joel and Thomson met at the convention, where the first told him that the heat was not conserved. Thomson was discouraged and later in his work “Towards a Dynamic Theory of Heat” suggested that in nature, apparently, there are two independent fundamental types of motion, then the works of Carnot and Joule should not contradict each other. Note that Kelvin also made a major contribution to telegraphy (to the problem of transmitting characters over long distances, invented a receiver, etc.).
The German physicist Gottlieb, known under the pseudonym of R. Clausius (1822-1888), in the monograph On the Driving Force of Heat (1850), introduced the concept of entropy * ; He assumed that in nature there are two fundamental principles of motion, he abandoned the idea of caloric acid, and explained the nature of heat by the behavior of particles of matter. In the 18th century, the Russian scientist M.V. Lomonosov.
The French physicist and engineer Clapeyron (1799-1864) made a significant contribution to thermodynamics. Note that in the years 1820-1830. Clapeyron worked in St. Petersburg at the Institute of Communications. During these years, he gave a mathematical form to the ideas of Carnot, introduced the diagram method of studying thermodynamic processes (PVT diagrams). In 1834, he derived the ideal gas state equation, which was subsequently generalized by D.I. Mendeleev (1870). He established the so-called Clausius – Clausius equations, which relate the boiling and melting points to the pressure of gases.
The third generation of thermodynamics opens Austrian physicist, Corresponding Member. St. Petersburg Academy of Sciences Ludwig Boltzmann (1844-1906). He established the connection between the thermal and mechanical forms of motion, showing that the basis of heat is the mechanical motion of atoms and molecules. Note that at that time the existence of atoms was not yet generally recognized. Boltzmann essentially developed the kinetic theory of gases and laid the foundations of statistical physics. Gibbs and Helmholtz belong to the same generation of thermodynamics.
American Gibbs (1839-1903) - prof. Yale University has developed chemical thermodynamics, i.e. made physical chemistry deductive science. Introduced the concept of free energy, showing how much energy can be obtained as a result of a chemical reaction. Introduced entropy diagrams in technical thermodynamics.
Hermann Helmholtz, German natural scientist, member of the Berlin Academy of Sciences and St. Petersburg Academy of Sciences (1821-1894), graduated from the Military Medical Institute and the University of Berlin, professor of physiology and physics at a number of universities. His research is related to electrodynamics, optics, heat, hydrodynamics. He also introduced into thermodynamics the concept of free and bound energy, gave the conservation law a universal character.
So, thermodynamics introduced the notion of probability into scientific use. However, this concept had arisen earlier in biology. For the living world in the XIX century, the English scientist Darwin discovered the basic law of its evolution, which was significantly different from the law of the evolution of the inert world. In the organic world, Darwin noticed the mechanism of evolution - natural selection.
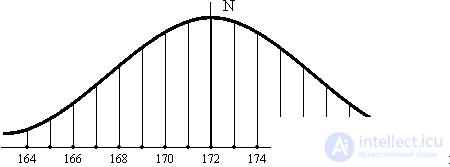
Each population has hereditary variation. The latter means a random deviation from the most likely average value of any characteristic of the organism. Consider the deviation from the average height in any large group of people (Fig. 1). Growth follows the law of the distribution of random variables: as one moves away from the average height of growth, the number of people N in a group decreases symmetrically. The same law obeys the velocity distribution of molecules in a gas. But unlike molecules, hereditary variability does not fade, like any fluctuation. Hereditary traits are fixed if they are of an adaptive nature, that is, they provide the mind with the best conditions for existence and reproduction. Random distribution evolves, changes. Physical nature also evolves in the direction of entropy growth S  S max if it is isolated. The arrow of time is directed towards equilibrium in an isolated system. When equilibrium is reached - events do not occur, time has stopped.
S max if it is isolated. The arrow of time is directed towards equilibrium in an isolated system. When equilibrium is reached - events do not occur, time has stopped.
So what is the difference between the evolution of the physical and biological worlds?
The physical world does not carry the memory of its evolutionary development; the biological world bears the memory of this. In biological systems, hereditary variability does not fade, as in physical ones, but inherits and reinforces those signs that allow one to survive. According to Darwin, the continuous birth of increasingly complex organized living forms, structures and systems occurs in the world. In the inert (physical) world, evolution leads for an isolated system to a state of equilibrium, i.e. to attenuation diversity. Biological theory speaks of the ubiquitous and continuous creation of Nature, and the inert world tends to destroy structures, leveling differences.
Both in the 19th and 20th centuries there was a dispute between two great theories of evolution.
In the middle of the 20th century, the famous physicist Schrödinger asked: maybe there are two types of laws - for living and inanimate matter? This question remained open until the 70s of the twentieth century, until a new teaching on evolution emerged, which found expression in synergy.
A brief expression of the above laws of the evolution of the biological world is contained in the Darwin triad * . Note that Darwin did not create an evolutionary theory, it was formulated earlier. His genius is that he first saw in nature the principle of natural selection and expressed it in this triad.
The French astronomer, physicist and mathematician P. Laplace (1749-1827) was the successor to the scientific affairs of Newton. His main works are devoted to celestial mechanics, contained in the five-volume work "Treatise on celestial mechanics", where the movement of the bodies of the solar system is explained on the basis of the law of world wideness, he proposed a hypothesis of the origin of the solar system. His physical research relates to molecular physics, heat, acoustics, electricity, optics. He is one of the creators of the theory of probability.
Let us briefly consider the views of Laplace on the problems of chance and regularity (determinism) in the development of the world, in particular, the laws of the motion of molecules. Molecules in a vessel move at random, colliding with each other. Their enormous amount (2.7 * 10 19 in 1 cm 3 of gas) and to follow the movement of each of them is impossible, therefore, to describe the properties of a gas, we use the averaged characteristics of molecules - the average speed  and from the equation
and from the equation  a temperature T and a bond of mechanical
a temperature T and a bond of mechanical  and thermal T parameters. Laplace believed that the movement of molecules is deterministic and there is no chance. Accident, statisticality is a subjective concept that reflects our ignorance. If there had been a certain demon (the Laplace demon) capable of determining the initial parameters of the molecules (coordinates and velocities), then, knowing the laws of motion, it would be possible to calculate in advance what will happen to the molecules (gas). In other words, chance does not objectively exist, behind it are deterministic laws. They know the demon Laplace, but we do not know them. Laplace wrote in "Experience the Philosophy of the Theory of Probability": "... we must consider the present state of the Universe as a consequence of its previous state and as the reason for the next ..." For a sufficiently extensive and informed mind, it is possible to embrace ... in one formula for the movement of the greatest bodies of the Universe along with the movement of the lightest atoms, nothing would remain that was unreliable for him, and the future, like the past, would appear before his eyes. "
and thermal T parameters. Laplace believed that the movement of molecules is deterministic and there is no chance. Accident, statisticality is a subjective concept that reflects our ignorance. If there had been a certain demon (the Laplace demon) capable of determining the initial parameters of the molecules (coordinates and velocities), then, knowing the laws of motion, it would be possible to calculate in advance what will happen to the molecules (gas). In other words, chance does not objectively exist, behind it are deterministic laws. They know the demon Laplace, but we do not know them. Laplace wrote in "Experience the Philosophy of the Theory of Probability": "... we must consider the present state of the Universe as a consequence of its previous state and as the reason for the next ..." For a sufficiently extensive and informed mind, it is possible to embrace ... in one formula for the movement of the greatest bodies of the Universe along with the movement of the lightest atoms, nothing would remain that was unreliable for him, and the future, like the past, would appear before his eyes. "
How will the fallen coin fall? Her behavior depends on how she was planted, on air resistance, and so on. The demon of Laplace can figure out which lot is drawn. The development of the universe follows the fatalistic laws, that is, everything in the world is determined, predetermined. From this point of view, entropy is a measure of our ignorance, a measure of a lack of information about the system.
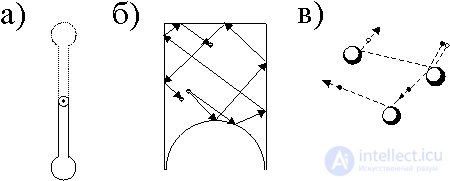
The problem of the correlation of mechanics and statistics leads to deep philosophical questions and was studied in detail in the middle of the 20th century by outstanding Russian scientists. In 1948, the physicist N. S. Krylov, studying the stable and unstable state of the pendulum, showed that not every solution of a mechanical problem can be compared with experience. In the lower position (Fig. 2a), the pendulum is stable, since small deviations will lead to small oscillations. The upper state of the pendulum is unstable, since the slightest push will drastically change the state of the pendulum. With strong instability, the trajectory of an individual particle is meaningless. It can be observed, but the calculation and experience will not be in agreement: the unstable trajectory "forgets" about its initial conditions. If the solution of a certain problem of mechanics is unstable, then it is actually not observable. They are implemented, that is, only stable solutions are observable. This means that an unstable mechanical process can become irreversible in time. Conventionally, sustainable systems can be considered isolated. In unstable systems (low impact leads to large deviations) the only way to consider small perturbations is statistical, based on their random nature. It is impossible to describe the behavior of such a system in the language of mechanics. It is necessary to introduce probability — the probability of one or another deviation in the motion of particles.
“Probabilistic description is more natural, it is not our ignorance that is expressed, but objectively existing instabilities, that is, objectively existing randomness of behavior. Owing to the impossibility of a complete description of such behavior of a mechanical system, irreversibility occurs. Incomplete description may be caused by various reasons: the incompleteness of the initial and (or) boundary conditions, the change of forces in the process of movement - the appearance of random forces "[1].
In the sixties of our century, to explain the emergence and development of instability, A. G. Sinai proposed a certain billiards - a vessel with one convex wall (Fig. 2, b). Two initially close trajectories further diverge indefinitely, that is, the process is unstable.
In fig. 2, c shows the passage of a beam through an inhomogeneous system consisting of arbitrarily arranged balls of different diameters.
So, Laplacian determinism is impossible: an attempt to predict the future, based on initial conditions and the laws of motion, immediately stumbles upon instability and ends in failure. Note that in mechanics, the trajectory of the body has a real meaning, but the values lacking content are due to probabilistic averaging: mechanical processes are adiabatic and  S = 0. On the contrary, in statistics they lose the meaning of the trajectory of bodies, but there are statistical characteristics of the system - temperature, entropy.
S = 0. On the contrary, in statistics they lose the meaning of the trajectory of bodies, but there are statistical characteristics of the system - temperature, entropy.
Consequently, entropy is really an objective measure of our ignorance, a measure of the lack of information about the system. This is a measure of the fundamental impossibility of knowledge determined by the instabilities of the trajectories ... The lack of information is a property of the system, and not a property of the observer.
Interesting criticism of Laplace determinism from the standpoint of the doctrine of ethics, which is given in the writings of the Russian natural scientist Timofeev-Resovskii. If Laplace determinism is true, then freedom of conscience, freedom of opinion does not exist: any true statement is already contained in a certain formula of the world. Practical activity is devoid of meaning - society has nothing to strive for, since everything is predetermined by a single formula. New physics gives freedom of conscience. This is one of the main results of natural science of the twentieth century.
The threat of thermal death of the universe was expressed in the middle of the nineteenth century. Thomson and Clausius, when he formulated the law of increasing entropy in irreversible processes. Heat death is a state of matter and energy in the Universe, when the gradients of parameters that characterize them have disappeared. Boltzmann, who discovered the connection between the entropy S and the statistical weight * P, considered that the present inhomogeneous state of the Universe is a grandiose fluctuation * , although its occurrence has a negligible probability. Boltzmann’s contemporaries did not recognize his views, which led to harsh criticism of his work and, apparently, led to the painful state and suicide of Boltzmann in 1906.
The current state of science is also inconsistent with the assumption of the thermal death of the universe.
First of all, this conclusion relates to an isolated system and it is not clear why the Universe can be attributed to such systems.
Further, in the Universe, a field of Stars and Galaxies acts, which Boltzmann did not take into account, and it is responsible for the appearance of Stars and Galaxies: the forces of formation of a structure from chaos, can generate Stars from Cosmic dust.
But this is a look at the problem in question from the twentieth century. It is interesting that long before that, in the general theory of relativity, the role of gravity in the structuring of chaos was revealed, in the poem of the Norwegian poet and prose Ibsen Star in the Fog in 1896 there were the following lines:
"A message was brought to earth What is where infinity stretched, The silent chaos became a star, That there are laws of there.
Now in the North fog swirls, Let him chaotic today, But there is a law in it, And therefore, the Star will light up here. "
Summarize the topic.
First of all, it should be noted the difference in views on evolution in the XVIII and XIX centuries. Classical science (Newton, Laplace) considers randomness as something external and irrelevant. Processes in the world were presented as reversible in time, predictable and retrospective for indefinitely large periods of time. Evolution is a process devoid of deviations, returns, side lines. The first blow to the views on evolution was dealt by thermodynamics and the evolutionary theory of the living world. Thermodynamics introduced randomness into science and considered it as an objective concept.
XIX century gave science two great theories of evolution for the inert and living world. In an inert world, development proceeds unidirectionally, towards the growth of entropy, i.e., to the alignment of the diversity of forms, gradients, etc. In the living world, on the contrary: development leads to an increase in the diversity of forms, that is, to an increase in the order and decrease in entropy. Only at the end of the 20th century will a single look at evolution be developed.
The Nobel Prize laureate I. Prigogine noted that the picture of the world of classical science of the XVIII century - Laplace determinism looks from a modern point of view almost like a "caricature of evolution."
The 19th century is remarkable not only for advances in thermodynamics, but also in other sciences. For example, ideas about forms of matter have been expanded. In the 18th century, only one form of existence of matter was known - the corpuscular one, which has clear outlines in which the body is concentrated. In the XIX century, the concept of the field form of matter was introduced. The introduction of the latter into the everyday life of science is associated with advances in the study of the phenomena of electricity, magnetism and electromagnetism. The nature of these phenomena was studied during the first half of the 19th century by a group of experimental scientists (Om, Biot, Savar, Faraday, and others) and summarized in the second half of the century in Maxwell's electromagnetic field theory. The XIX century is also remarkable in other areas of science, but we are not talking about them now.
The further development of thermodynamics is interesting. During the 19th century, the basic principles (principles) of thermodynamics of isolated systems were formulated. In the first half of the 20th century, thermodynamics developed mainly not deep down, but in breadth, its various sections appeared: technical, chemical, physical, biological, etc. thermodynamics.Only in the forties there appeared works on the thermodynamics of open systems near the equilibrium point, and in the eighties synergetics appeared. The latter can be interpreted as the thermodynamics of open systems far from the equilibrium point.
Comments
To leave a comment
Synergetics
Terms: Synergetics