Lecture
Recall some fragments from the field of physical optics, while starting with the mechanisms of emission and absorption of matter. For individual substances (gases, liquids, glasses, etc.), the emission and absorption of radiation energy by the entire volume of a substance is characteristic.
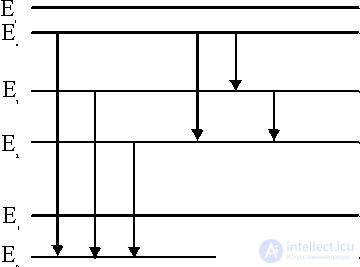
Figure 1. Diagram of energy levels.
Consider the physical mechanism of these processes for example radiating gas, which may consist of molecules, atoms, ions and electrons. The energy states of these particles are different and are usually described using a diagram (Fig. 1). There is a bound state when the atom is not ionized and free when the atom breaks up into an ion and an electron. The zero energy level E 0 corresponds to the ground state (the lowest of all bound states), and the higher bound states correspond to the energy levels E 1 , E 2 , etc. The E level corresponds to the ionization energy, and higher to E and to free electrons.
Radiation processes (absorption, emission, scattering) are conveniently described from the standpoint of quantum concepts, where the basic minimum unit of radiation energy is a quantum of radiation — a photon. The emission of radiation is the process of emitting photons, and the absorption is the capture of a photon by a particle, the dispersion is the change in the trajectory of a photon as it passes around the particle. The transition from the energy state, for example, E 3 to the state E 2 is accompanied by the emission of a photon with a frequency 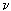 23 and energy h
23 and energy h 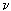 32
32
E 3 - E 2 = h 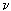 32 ,
32 ,
where h is Planck's constant.
The absorption of a photon is associated with the inverse process E 2 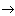 E 3 .
E 3 .
So, the transition between certain energy levels corresponds to a fixed frequency and, in the absence of other phenomena, the emission or absorption spectrum will have a ruled character.
Various types of transitions are possible: bound-bound, bound-free, free-free.
Bound-bound transitions correspond to a process in which an atom or molecule emits or absorbs a photon, but neither ionization nor recombination of ions and electrons occurs.
A bound-free transition is a process in which an atom absorbs a photon with energy sufficient for ionization. An ion and a free electron with any kinetic energy are formed. Associated free radiation forms a continuous spectrum.
A free-free transition (bremsstrahlung) occurs in an ionized gas: the electron passes near the ion and interacts with its electric field. An electron can absorb a photon or emit a photon, while its kinetic energy increases or decreases. The emission and absorption spectra will be continuous.
Consider the concept of stimulated or stimulated radiation. Let the atom be in an excited state and can emit photons. If this atom is placed in the field of external radiation, which already contains photons with such energy, then the probability of radiation of a photon increases - this is stimulated radiation. The emitted and external photons are in the same phase, and their trajectories coincide.
We offer readers to get acquainted with the mathematical model of the laser.
Comments
To leave a comment
Synergetics
Terms: Synergetics