Lecture
The concept of production and entropy flow in open systems is given. It is shown that for such a system, a decrease in entropy is possible in principle. To describe such processes, a dissipation function is introduced, depending on the energy flux and the force causing it. Irreversible processes in open systems are considered. All theoretical positions are illustrated by concrete examples - an astronaut in a spacecraft, a person in a steam room, cell division.
I. The system itself (d i S) and the environment (d e S), contributes to the total entropy change of the open system dS, i.e.
dS = d i S + d e S
Ii. Synergetics is based on the position that the change in entropy in an open system can be less than zero, namely: dS <0, if d e S <0, but | d e S | > d i S; and then spontaneous development of events from chaos to order is possible (a consequence of I)
Iii. The processes occurring in open systems can be described using the dissipation function (entropy production per unit time per unit volume), which introduces time which was absent in thermodynamics.
Iv. The dissipation function can be defined for processes studied in various fields of science as the product of the flow of energy by the force that causes it.
V. In conjugated systems, for example, of two components, the energy flow in the first system is the same as in the second (both are caused by the corresponding forces), - Onsager's theorem.
Vi. If several irreversible processes take place simultaneously, then in an open non-equilibrium system, these processes are associated with each other, and can lead to the appearance of new effects. For example, when thermal and electrical conductivity is applied, thermoelectricity appears, and diffusion and thermal conductivity - thermal diffusion.
We now consider the behavior of open systems * that are exchanged with the environment and matter and energy. The change in entropy * of such a system consists of internal changes in d i S entropy and its inflow or outflow into the system d e S due to heat exchange with the environment and as a result of the exchange of matter. Therefore, the total entropy change of the open system dS is equal to
dS = d i S + d e S (1)
We show that the sign of the production of entropy d i S 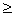 0. To do this, we surround the system with an adiabatic * shell, then the inflow or outflow of matter and energy will cease, that is, de S = 0. According to the second law of thermodynamics dS
0. To do this, we surround the system with an adiabatic * shell, then the inflow or outflow of matter and energy will cease, that is, de S = 0. According to the second law of thermodynamics dS 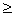 0, formula (1) for such a system takes the form dS = d i S
0, formula (1) for such a system takes the form dS = d i S 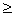 0, i.e., d i S is always greater than or equal to zero.
0, i.e., d i S is always greater than or equal to zero.
The sign d e S can be either positive or negative, depending on the specific conditions, i.e., the influx of entropy can be more or less of its outflow. Therefore, according to formula (1), the entropy change in an open system can be both greater and less than zero.
Consider the possible situations:
d e S> 0, d i S 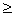 0 always, dS> 0; 0 always, dS> 0; |
 |
| d e S <0, but | d e S | i S, dS> 0; | |
| d e S <0, but | d e S | > d i S, dS <0; | |
| d e S <0, but | d e S | = d i S, dS = 0. |
Attention should be paid to the last two cases: in an open system, a state is possible when the entropy does not change or even decreases , that is, the principal possibility of spontaneous movement from chaos to order is shown. This conclusion is fundamentally new and opens up interesting perspectives in the study.
Let there be no entropy production in the system, i.e. d i S = 0, but there is only a heat flow, then
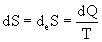 ,
,
With the simultaneous production of entropy, the formula (1) takes the form
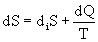 .
.
The second law of thermodynamics teaches us that all natural processes are accompanied by an increase in entropy. If we consider two processes (entropy production and entropy flow) in an open system with interprocess communication, then we can see that the unnatural move is dS <0. A simple illustration of this principle is shown in the figure.

Fig.1.Dva cargo: a) - not related b) - interconnected
Two unrelated loads tend to fall down (a); however, if they are connected by a pulley, then the lighter ones will rise due to the heavy (b). This is a kind of mechanical analogue of the phenomenon under consideration about the "unnatural" course of the process.
Comments
To leave a comment
Synergetics
Terms: Synergetics