Lecture
Membrane proteins
The polypeptide chain is similar to a string of beads (Fig. 1-5 A): each “bead” is an amino acid. Moreover, since amino acids can rotate around peptide bonds, the polypeptide chain is able to bend and fit in various forms, like a string of beads, which can be twisted into many configurations. The three-dimensional shape of the molecule is known as its conformation. The conformations of peptides and proteins play a major role in their functioning.
Four factors determine the conformation of the polypeptide chain after the amino acid sequence is formed:
• hydrogen bonds between chain sites or between chain sites and surrounding water molecules;
• ionic bonds formed between polar and ionized sites along the chain;
• van der Waals forces, which are very weak attractive forces between closely spaced non-polar (hydrophobic) areas;
• covalent bonds between the side chains of two amino acids.
Since peptide bonds are located along a chain at regular intervals, hydrogen bonds between them tend to impart a spatial organization to the chain, known as the α-helix. Hydrogen bonds can also form between peptide bonds when the protruding portions of the polypeptide chain run roughly parallel to each other, forming relatively straight structures like the folded layer, which are known as the β-layer. However, for various reasons, these regions of polypeptide chains may not form conformations in the form of α-helix or in the form of β-layer. For example, the size of side chains and ionic bonds between oppositely charged side chains can prevent the formation of repeating hydrogen bonds necessary for the formation of these structures. These unordered
portions, called loops, are found in places connecting more regular α-helices and β-structural regions.
Covalent bonds between certain protein chains can also bend regular folded layers. For example, the amino acid side chain of cysteine contains a sulfhydryl group (R-SH), which can interact with the sulfhydryl group in the other side chain of cysteine, forming a disulfide bond (RSSR) that binds two amino acid chains together. Such disulfide covalent bonds are formed between sites of the polypeptide chain as opposed to weaker hydrogen and ionic bonds, which are more easily destroyed. The same bonds are involved in other intermolecular interactions described below.
Most proteins consist of not one, but several polypeptide chains, and they are called multimeric (oligomeric) proteins. The same factors that affect the conformation of a single polypeptide determine the interaction between polypeptides in oligomeric proteins. Thus, the chains can be maintained together due to the interaction between different ionized, polar and non-polar side chain radicals, as well as through the formation of disulfide covalent bonds between the chains.
The primary structure (amino acid sequence) of the overwhelming amount of proteins is known, but three-dimensional organization is defined only for a very small number of proteins. Due to the fact that many factors can alter the packaging of the polypeptide chain, it is currently not possible to accurately predict the spatial organization of a protein based on its primary structure.
In fig. 1-5 B and fig. 1-5 V are models of integral proteins incorporated into the membrane.
In fig. 1-5 G shows the standard designation of the integral protein, and Fig. 1-5 D duplicates fig. 1-5 V, but in the form of a standard notation.
Fig. 1-5. Schematic representation of various proteins that are differently embedded in the membrane.
A is a schematic diagram of a polypeptide molecule embedded in a lipid bilayer. Known symbols denote amino acids that make up the protein. B - model of a polypeptide molecule embedded in a lipid bilayer. Amino acids are indicated by globules. B - models of polypeptide molecules embedded in a lipid bilayer. Transmembrane fragments of each molecule are labeled with spirals. G - the main model used polypeptide molecules embedded in the lipid bilayer. The transmembrane fragment of the molecule is designated by a cylinder. D - models of polypeptide molecules embedded in the lipid bilayer. Transmembrane fragments of each molecule are marked with cylinders.
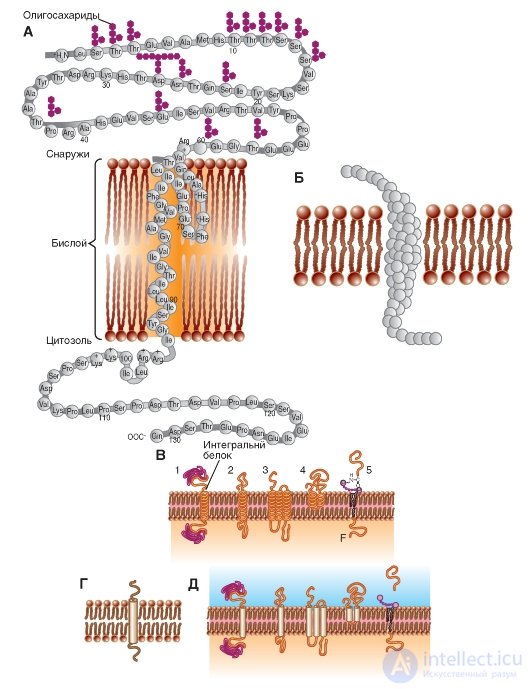
Integral membrane proteins in the membrane model
All membranes, in addition to phospholipids, contain proteins. Although J. Danielle and H. Dawson, when discussing how to bind a protein with a double layer of lipids, used the term “mosaic”, they rejected the suggestion that the stretched protein film covers one or another side of the membrane lipid bilayer. The idea of stretched protein molecules that are in the β-layer configuration has become part of the “elemental membrane” hypothesis put forward by J. Robertson. However, it was further shown that the membrane protein is predominantly in the form of an α-helix, rather than a β-layer. It is now recognized that there are proteins and membranes linked to both sides, and penetrating through the double lipid layer.
Depending on the method used to separate proteins from membranes, membrane proteins belong to one of two large groups. The first group is peripheral, or external, proteins, which pass into the supernatant when the membranes are washed with buffer solutions with different pH or ionic strengths, or solutions containing complexing agents, such as EDTA or EGTA. The second group - integral, or transmembrane, proteins that retain communication with the membranes and after these operations; therefore for their release it is necessary
first destroy the structure of the phospholipid bilayer.
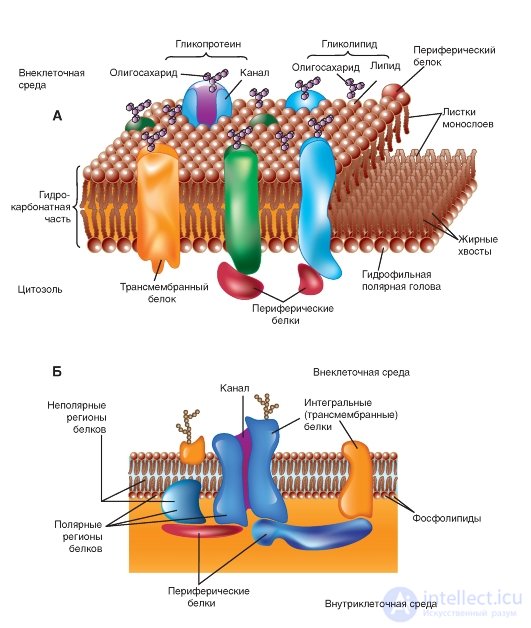
In 1972, S. Singer (S. Singer) and G. Nicholson (G. Nicolson) were the first to describe integral proteins (Fig. 1-6 A, B). At present, it is shown that these proteins are extremely diverse in their structure. They are asymmetrically distributed in the bilayer. The overwhelming majority of integral proteins repeatedly cross the lipid bilayer - zigzag proteins. They perform many different functions. Integral proteins act as hydrolytic enzymes, cell surface receptors, redox components of the electron transport system, and as specific carrier proteins.
From histological studies it is known that in glycosylated integral proteins, the region containing carbohydrates is located either on the cell surface or inside the cavity of the endoplasmic reticulum or the Golgi complex.
Many integral proteins contain sequences of hydrophobic amino acids in their polypeptide chains; however, some are linked to the lipid bilayer by a different mechanism. A portion of the integral proteins are covalently bound to lipids.
Currently, a great interest of researchers is the study of membrane protein conformation. Many important processes are accompanied or caused by a change in the way the polypeptide chain is laid, that is, by a change in the conformation of protein molecules in the membranes.
Fig. 1-6. The membrane model. A lipid bilayer with proteins embedded in it performing various functions is shown.
Comments
To leave a comment
Human physiology, hygiene and age physiology
Terms: Human physiology, hygiene and age physiology