Lecture
Lipids, widely represented in the cells of the body, are molecules consisting mainly of carbon atoms and hydrogen. Since these atoms are bound by neutral covalent non-polar bonds, lipids are non-polar and have very low solubility in water, which is a characteristic feature of this class of organic molecules. The main subclasses of lipids are fatty acids, neutral fats (aka acylglycerols or glycerides), steroids. The most common membrane lipids are glycerol-substituted and sphingosine-substituted lipids, as well as glycolipids and representatives of steroids, sterols.
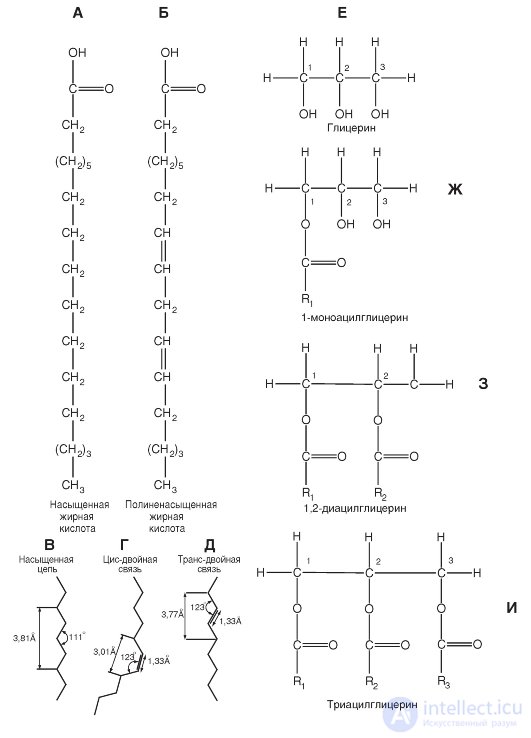
Fatty acid molecules are long carbohydrate chains with a terminal carboxyl group. A typical example of fatty acids is shown in fig. 1-1, A, B.
Due to the fact that fatty acids in the body are synthesized by combining two-carbon fragments, most fatty acids have an even number of carbon atoms, from 14 to 22.
If all the carbon atoms in fatty acids are bound by single covalent bonds, then this fatty acid is called a saturated fatty acid. Some fatty acids contain one or more double bonds, and then they are called unsaturated. If a fatty acid has one double bond, then it is called a monounsaturated acid. When there are more than one such bond, they are talking about polyunsaturated fatty acid.
Saturated and unsaturated fatty acids differ in their structural configuration. In saturated fatty acids, hydrocarbon
the tail can take on many conformations due to the freedom of rotation around each single bond (Fig. 1-1, B), however, the configuration of the elongated form as the most energetically most favorable is most likely.
In unsaturated fatty acids, rotation around the double bond is impossible, and this causes a rigid bending of the hydrocarbon chain (Fig. 1-1, D, D). In unsaturated fatty acids, the cis- double-bond configuration creates a bend in the aliphatic chain at an angle of about 30 ° (Fig. 1-1, D). In the trans form of the double bond, the hydrocarbon chain conformation differs little from the saturated chain conformation.
(fig. 1-1, D).
Glycerol fatty acid esters are called neutral fats, acylglycerols or glycerides. They constitute the main component of fat stored in the cells. The structural basis of these lipids is a substituted trihydric alcohol glycerol (Fig. 1-1, E).
If all three hydroxyl groups of glycerol are esterified with fatty acids, then this compound is called triacylglycerol (Fig. 1-1, I). The three fatty acids in the triacylglycerol molecule are different, and thus different fats may include fatty acids with chains of different lengths and different degrees of saturation. Hydrolysis of triacylglyrides leads to the release of fatty acids from glycerol, and these products can be further decomposed with the release of energy necessary for the functioning of cells. Triacylglycerols are the bulk of natural neutral fats. In addition to triacylglycerols, diacylglycerols are found (Fig. 1-1, 3) and monoacylglycerols (Fig. 1-1, G).
Fig. 1-1. The main compounds that form phospholipids.
A and B are examples of fatty acid molecules. Fatty acid molecules are long carbohydrate chains with a terminal carboxyl group. (A) saturated (all carbon atoms in a fatty acid are linked by single covalent bonds) and (B) polyunsaturated (contain one or more double bonds) fatty acids are shown. Next, panels C, D, D show the configuration of the fatty acid bonds: saturated (C), unsaturated with cis bond (D), unsaturated with trans-double bond (D). E - substituted trihydric alcohol glycerin, the structural basis of lipids. (G) 1-monoacylglycerol. (3) 1,2-diacylglycerol as the basis of the phospholipid. In position 2 is usually an unsaturated fatty acid. (I) Triacylglycerol. The positions of the fatty acids are marked as R 1 R 2 and R 3
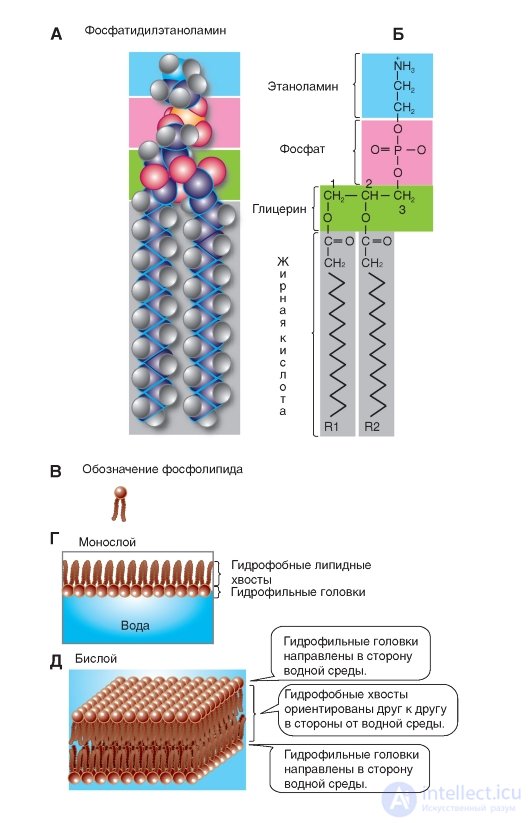
The structural basis of glycerol derivatives is a substituted trihydric alcohol glycerin. Phospholipids of membranes are represented mainly by phosphoglycerides (Fig. 1-2 A, B). Interestingly, these compounds in the body are found almost exclusively in biological membranes. In phosphoglycerides, one of the primary hydroxyl groups of glycerol (group 3 in Fig. 1-2 B) is esterified with phosphoric acid, not fatty. In other words, the basis for their construction is not glycerol, but glycerophosphoric acid. If in position 3 of glycerin there is a phosphoric acid residue, then the corresponding compound is called phosphatidyl acid.
In addition, in some cases a small polar ionized nitrogen-containing molecule is added to this phosphate, for example, as in phosphatidylserine and phosphatidylcholine (Fig. 1-2 B). These groups constitute the polar (hydrophilic) region at one end of the phospholipid. The other two hydroxyl groups of glycerol (groups 1 and 2) are fatty acid residues and ensure the formation of a non-polar (hydrophobic) region at the opposite end of the molecule.
Thus, the molecules of all phosphoglycerides contain a polar head and two non-polar hydrocarbon tails. Therefore, they are called amphipathic, i.e., combining properties and hydrophilicity and hydrophobicity. In water, they are organized into clusters with polar ends that attract water molecules.
Phosphoglycerides differ from each other mainly in the X-group of the polar head of the molecule. The simplest type of phosphoglyceride is phosphatidic acid, which does not have X-groups. In cells, it is contained in a small amount and is an intermediate product in the biosynthesis of other phosphoglycerides.
Based on the above structure, phospholipids are usually designated as shown in Figure 1-2 B.
The properties of natural membranes are often examined in their models, which are artificial phospholipid membranes. The technique of artificial lipid membranes was developed by Langmuir in 1917. If a drop of phospholipids or fatty acids dissolved in any volatile solvent is applied to the surface of the water, after the molecules are distributed over the water surface and the solvent evaporates, a monomolecular film is formed. As Langmur established, with the full saturation of the surface layer, the adsorbed lipid molecules are perpendicular to the water surface in such a way that the hydrophilic polar group is immersed in the water, and the non-polar hydrocarbon chain is directed vertically upwards. Such an oriented layer of molecules is called the “Langmuir palisade” (Fig. 1-2 G).
If the glass plate is immersed in water, on the surface of which there is a monomolecular lipid film, then this film can be transferred to the surface of the plate. When re-immersing on the plate, bimolecular films appear (Fig. 1-2 D).
Fig. 1-2. Phospholipids and membrane formation principles.
A, B - membrane phospholipids are mainly represented by phosphoglycerides. In phosphoglycerides, one of the primary hydroxyl groups of glycerol (group 3) is esterified with phosphoric acid, not fatty, that is, their base is not glycerol, but glycerophosphoric acid. A polar ionized nitrogen-containing molecule is added to the phosphate, as shown for phosphatidylethanolamine. This group constitutes the polar (hydrophilic) region at one end of the phospholipid. The other two hydroxyl groups of glycerol (groups 1 and 2) are fatty acid residues and ensure the formation of a non-polar (hydrophobic) region. In - the designation of the phospholipid. G - a monolayer of phospholipids on water. D - bilayer phospholipids
Comments
To leave a comment
Human physiology, hygiene and age physiology
Terms: Human physiology, hygiene and age physiology