Lecture
The currently accepted classification divides all K + channels into:
• potential-controlled K + channels (K V );
• Ca 2+ - activated K + channels (K Ca );
• K + channels of anomalous rectification with inbound rectifier current (K ir );
• K + channels with two loops in the domain (two-P K + -channels) (К 2Р ).
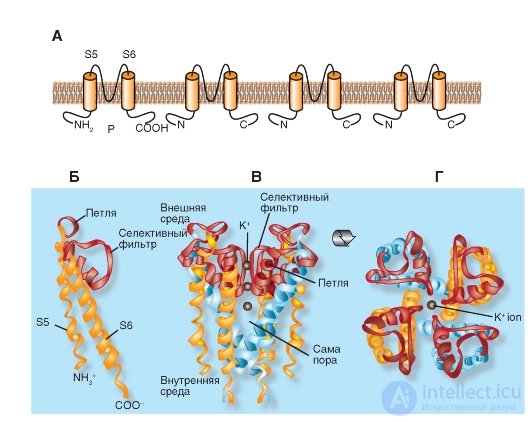
We begin the description of the molecular organization of K + channels with the simplest form presented, for example, in the bacteria Streptomyces lividans. The model of the molecular organization of such the simplest K + channel of the Streptomyces lividans bacterium is shown in Fig. 1-13.
Shown in fig. 1-13 And К + -channel of bacteria consists of four identical subunits united in a single whole channel body.
Panels B, C and D show that the P-loop is localized near the extracellular surface of the membrane and combines the α-helices of both S5 and S6 segments. It consists of a non-helix part forming the upper part of the pore, a short α-helix and an extension loop that protrudes into the tapering part of the pore and forms a selective filter. This filter allows only K + ions, but not other ions, to pass through the channel. Below the filter is the central part of the pore formed by the α-helix of the S6 segment. In the tetramer K + channel, the bacteria of Streptomyces lividans S5 and S6 are labeled as they are structural equivalents of the S5 and S6 segments in the potential-controlled K + channel (Fig. 1-13).
Figure 1-13 B shows the structural details of the channel. The NH 2 end of the subunit begins with an outer helix (orange), which penetrates the membrane from the cytoplasmic side to the outer surface. The outer helix is followed by a short helix (red) directed into the pore. Then follows the internal spiral, which returns to the cytoplasmic side (orange). The connecting loop between the outer and short spirals forms a structure that forms the outer opening of the pore. This part contains the binding center for the non-selective blocker of K + channels of tetraethylammonium (TEA). Four such loops of each subunit form the outer walls of the pore, namely, that part of it that is responsible for the selectivity of the channel - a selective filter.
The channel selectivity is achieved both by the size of its pores and the molecular organization of the selective filter. The diameter of the selective filter is approximately 0.3 nm. Amino acids of its wall are oriented so that successive rings formed by four carboxyl groups (one from each subunit) are turned inside the pores. The pore diameter is sufficient to pass a dehydrated K + ion having a diameter of 0.27 nm. Oxygen atoms in the pore wall replace ion hydration. Smaller ions, such as Na + , with a diameter of 0.19 nm, cannot pass through the K + channel, since they cannot form a close bond simultaneously with all four oxygens of the pore walls. This mechanism is presented in detail in Fig. 1-19.
Fig. 1-13. Model of molecular organization of the most simple K + channel of the Streptomyces lividans bacterium .
And this K + channel represents a tetramer of identical subunits, each of which contains two similar transmembrane α-helices, denoted as S5 and S6, and a short P-loop forming a pore. B - the organization of one of the four subunits. S5 and S6 α-helices (yellow in the figure) and loop P (pink) are shown. B - tetramer channel organization. Side view. G - tetramer organization of the channel. View from above
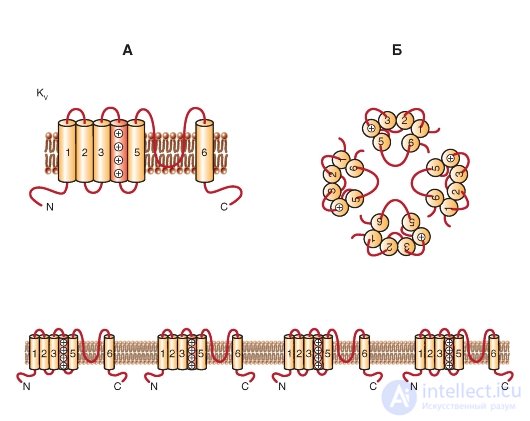
KV channels are formed by two types of protein subunits: α-subunits, which form the pore, and auxiliary β-subunits. Α subunits form a tetramer to form a functional channel that conducts K + ions.
One subunit of the Κ V- channel is a protein with six transmembrane segments (S) of the α-helix (S1-S6) and N-and C-terminals located in the cytoplasm. The most studied are the extracellular loop P between 5S and 6S (it forms the K + -selective filter and the pore channel) and 4S, which is positively charged and forms a protein voltage sensor (Figure 1-14 A). In addition, many functions are usually attributed to large N- and C-terminal loops. They include the control of channel inactivation, protein-protein interaction (like tetramerization of α-subunits, interaction with cytoplasmic β-subunits, and preservation of sequences).
K V α-subunits can form either homotetramers (homotetramers) or heterotetramers (heterotetramers), probably limited in the same subfamily. The enormous diversity of K V -currents in vivo is a consequence of the existence of splicing variants (gene isoforms encoding the same α-subunit), assembling of the α-subunit tetramer with different
auxiliary subunits and heteromerization of α-subunits.
As is known, K + ions are unequally distributed between the extracellular medium and the cytoplasm, and this creates a driving force for the release of K + ions in the large physiological limits of the membrane potential. The V- channels are open when the transmembrane potential depolarizes, which contributes to the release of K + ions. Depolarization can be represented as the accumulation of positive charges on the outer side of the membrane. However, the yield of positively charged ions, such as K + ions, shifts the membrane potential below the resting potential. Thus, the K V- channels feel depolarized and, in turn, act to eliminate it. One of the most characterized roles of K V -channels is to end the strong depolarization caused by the activation of the potential-dependent entry of cations (together forming the action potential). Thus, it can be postulated that any increase in K V- channel activity will lead to a more effective end to depolarization (usually shortening or removing the action potential). This functional ability of K V channels also underlies the formation of bursting activity (explosive). Thus, the activity of K V- channels plays, for example, a role in coding information in the nervous system.
Fig. 1-14. Planometric model of the molecular organization of a potential-controlled K + channel (K of the V channel).
A - one subunit of the K V- channel is an α-helix with six transmembrane segments (S1-S6) and N- and C-terminals located in the cytoplasm. The extracellular P-loop between the S5 and S6 segments forms a selective filter for K + ions and the channel pore, and the positively charged S4 segment forms the protein voltage sensor. Long N- and C-terminal loops control channel inactivation and protein-protein interactions. B - association of four subunits in the K V- channel
So far, 38 genes have been described in the human genome encoding various channels of the K V superfamily. V- channels include classical channel categories: K + -channels of delayed rectification (delayed rectifier K + -channels) and fast transient K + -channels of the outgoing current (fast transient K + -channels: fast transient K + -current I A , or transient outward current i to ).
The name "delayed rectifier K + -channels" was originally given to the potential- controlled K + - channels of the giant squid axon due to their delayed activation (as compared with the rapidly activating Na + channels). Members of all K V subfamilies (including K V 1-4, EAG and KCNQ) may form delayed rectifier K + -channels.
Fast transient K + -channels (forming fast transient K + -current: I A , or transient outward current I to ) - fast transient ^ -channels of the outgoing current are low potential activated channels (low voltage-activated). They are rapidly inactivating (transient, otherwise temporary, K + channels). Channels of this type are usually formed from members
K V 1 and K V 4 subfamilies and an auxiliary β-subunit, often necessary for the phenomenon of rapid inactivation. Recently, a new auxiliary subunit, CD26, related to the dipeptidyl amino peptide-like protein (Dipeptidyl Aminopeptidase-like protein, DPPX) was discovered, and this allowed us to assign a K V 4-channel characteristic to the neuron channel.
Phylogenetic trees of K V -channels are shown in Fig. 1-15. During phylogenetic reconstruction based on a similar amino acid sequence, it shows the potential- controlled (voltagegated) K + channels of the K V 1-K V 6 families, K V 7 and K V 8-K V 9. Among this group, only K V 1.8 in currently lacks the name HGNC. Five members of the K V group of the K V 7 family (KCNQ1-KCNQ5) can now (from 2005) be affiliated with other proteins of the K V channel and therefore not shown in a separate phylogenetic tree, as was previously thought. The three remaining families K V - K V 10, K V 11 and K V 12 are closely related to each other to be shown in a separate phylogenetic tree.
Fig. 1-15. Phylogenetic trees for the family K V.
A is a phylogenetic tree for the K V 1-К ^ 9-channel family. Amino acid sequences of the K V channel include the K V 1-K V 6 and K V 8-K V 9 families that were introduced before 2005. The B - K K sequences 7.1-K V 7.5, K V 6.4 and K V 8.2 were added to existing phylogenetic tree. Only the hydrophobic cores of the channel (S1-S6) were used for analysis. B - phylogenetic tree for families of K V 10-K V 12-channels

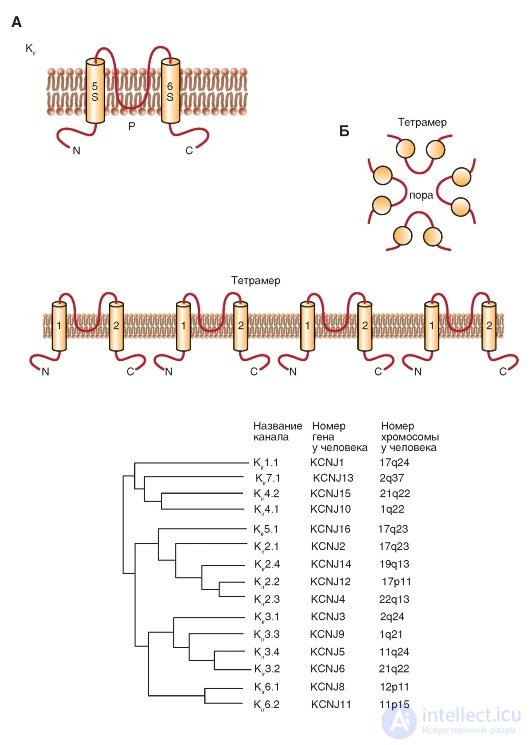
The next class of 2S proteins includes three groups of inward rectifiers channels (K ir ), which can be called, although very long, as “K + channels of abnormal rectification with a current of the incoming direction”. The N and C terminals of these channels are also located in the cytoplasm, the P-loop between the two transmembrane domains forms the pore, and the functioning channel is the tetramer of these 2S / 1P subunits.
The first inward rectifiers K + -channels - K ir 1.1 (ROMK1) and K ir 2.1 (IRK1) - were cloned in 1993, and the sequence of new members of this family was identified, including the K-channel associated with the G-protein, (K ir 3) and ATP-sensitive K-channel (K ir 6). These channels play an important physiological role in the function of many organs, including the brain, heart, kidneys, endocrine cells, auditory cells and retinal cells. Molecular organization of these channels is shown in Fig. 1-16 A. The phylogenetic tree shown in fig. 1-16 B, illustrates related relationships, based on the sequence of amino acid residues, between the seven main
subfamily K ir . Currently, new members of this family have not been identified. Since this phylogenetic tree appeared only in 2002 in the IUPHAR edition, it is unlikely that any other new members were discovered during this time.
Recently, the X-ray crystallography method has made it possible to describe the structure of the cytoplasmic region of K ir 3.1, the complete structure of the bacterial K ir 1.1 and the cytoplasmic region of K ir 2.1. These data demonstrate that inward rectifiers K + -channels have a long pore extending into the cytoplasm, and emphasize the importance of negative charges of amino acid residues on the wall of the cytoplasmic part of the pore, which plays an important role of abnormal straightening. These studies provided an insight into the mechanisms of channel gate regulation through ligands through G-proteins and phosphatidylinositol 4,5-bisphosphate. The information obtained through the analysis of the crystal structure is extremely valuable, as it allows us to evaluate structural and functional relationships. Also noteworthy are publications devoted to the study of the dynamic aspects of the channel function.
Fig. 1-16. Molecular organization of inwardly rectifying potassium channels - K + channels of anomalous rectification with a current of the incoming direction (K ir ).
A - one subunit of the K 1r channel is an α-helix with two transmembrane segments (S5 and S6), N-and C-terminals located in the cytoplasm, and a short P-loop forming a pore. B - association of four subunits in the K ir channel. B - phylogenetic tree for K ir channels
Comments
To leave a comment
Human physiology, hygiene and age physiology
Terms: Human physiology, hygiene and age physiology